Waste Vegetable Oil As A Diesel
Replacement Fuel
 Index of Waste Vegetable Oil As A Diesel Replacement Fuel Index of Waste Vegetable Oil As A Diesel Replacement Fuel
 Introduction Introduction
 Properties of Triglycerides as Fuels Properties of Triglycerides as Fuels
 Oils and their melting point and Iodine Values Oils and their melting point and Iodine Values
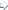 In Car installation In Car installation
 Problems Costs Exhaust emissions and Cost efficiency Problems Costs Exhaust emissions and Cost efficiency
 Saturated oils and possible improvements Saturated oils and possible improvements
Oils and their melting point and Iodine Values
Oil Approx. melting Iodine
point ° C Value
Coconut oil 25 10
Palm kernel oil 24 37
Mutton tallow 42 40
Beef tallow 50
Palm oil 35 54
Olive oil -6 81
Castor oil -18 85
Peanut oil 3 93
Rapeseed oil -10 98
Cotton seed oil -1 105
Sunflower oil -17 125
Soybean oil -16 130
Tung oil -2.5 168
Linseed oil -24 178
Sardine oil 185
All of these problems can be at least partially alleviated by dissolving the oil or
hydrogenated oil in petroleum diesel. ‘ Drying oils’ such as linseed oil for
example, could be mixed with petroleum diesel at a ratio of up to about 1:8 to
give an equivalent IV in the mid-twenties. Likewise coconut oil can be thinned
with diesel or kerosene to render it less viscous in cooler climates. Obviously the
solubility of the oil in petroleum also needs to be taken into account.
Another method is to emulsify the oil or fat with ethanol. Goering [12] found that
eight parts of soybean oil, when emulsified with two part ethanol and five parts of
1-butanol as stabiliser, performed as well as diesel fuel and was able to start a
cold engine. The cost was calculated (in 1981) to be $0.40 a litre as compared to
$0.30 – 0.35 per litre for diesel.
Trans-esterifying triglyceride oils and fats with monohydric alcohols to form
biodiesel largely eliminates the tendency of the oils and fats to undergo
polymerisation and auto-oxidation and also reduces the viscosity of the oil to
about the same as petroleum diesel.
However as previously mentioned, the ‘ back-yard’ production of use of does
pose some risks, particularly to those who are not familiar with the handling of
toxic and highly flammable liquids.
In many cases, it is possible to use a variety of triglyceride fats and oils as a fuel.
While engine wear and maintenance may be increased, in some circumstances
these problems are not serious enough to prevent the use of the triglycerides as
a fuel.
Conversion of a vehicle to operate on Waste Cooking Oil
An alternative to the use of biodiesel is the use of vegetable oil or animal fats as
a fuel. The differences amongst fats and oils, whether of animal or vegetable
origin, relate mainly to the level of saturation in the carbon chain. Generally
speaking, the lower the number of double bonds, the higher the melting point of
the triglyceride and the greater the stability of the triglyceride to polymerisation
and spontaneous oxidation. From an engine use point of view, it is preferable to
use saturated fats as fuels as they are more stable and less resistant to
oxidation, particularly under the elevated temperatures and pressures as found in
an engine environment. However due to their higher melting points, difficulty may
be encountered in starting the engine without pre-heating of the fat.
In order to test the viability of using relatively unsaturated oils in engines, a 1990
Mazda with a 2.0 litre indirect injection OHC diesel was obtained with a view of
running it on various types of triglyceride oils and fats. At that time of purchase,
the vehicle had covered 222,000 km.
The previous owner stated that the engine head had been overhauled, but no
further details were provided. Since the purchase, and prior to the conversion to
operate on triglyceride oils, the injector pump was overhauled. Fuel consumption
of the vehicle on diesel was stable at about 6.9 L/100 km.
The vehicle is used as a family vehicle in a 2-car household. At the time of
conversion (October 2000), the vehicle had covered 231,000 km.
Waste palm oil (a solid fat) was used initially but the time delay in melting this oil
prevented use of the oil on short journeys. Consequently, waste canola oil was
tried and has been used exclusively for the last 7,500 km.
At about 80 cSt (at 20° C), the viscosity of used canola oil is significantly greater
than that of diesel which has a viscosity in the range of 2 to 4.5 cSt.
However, as canola oil is warmed, its viscosity falls quite significantly and at
about 70° C the viscosity is about 5 to 10 cSt. Thus the viscosity is sufficiently
low to allow its use as a replacement fuel for diesel with out too much difficulty.
Table 2 Comparison of properties of diesel, canola oil and commercial US
biodiesel.
Diesel Canola Oil Biodiesel
Density kgL-1 @ 15.5° C 0.84 0.92 0.88
Calorific value MJL-1 38.3 36.9 33 – 40
Viscosity mm2s-1 @ 20° C 4 - 5 70 4 – 6
Viscosity mm2s-1 @ 40° C 4 - 5 37 4 – 6
Viscosity mm2s-1 @ 70° C 10
Cetane number 45 ~ 40 - 50 45 – 65
The vehicle was fitted with an additional 17 litre fuel tank under the bonnet
together with the necessary fuel lines, additional filter and a solenoid valve to
control the fuel source. Electrical connections to a thermostat, glow plug, run-on
timer, switches and the solenoid valve were also installed.
<< last page - index - next page >>

|
![]()